Air Pressure Curriculum
Introduction
Challenges in Understanding Air Pressure
Pressure is defined as the force per unit of surface area, where the force is applied perpendicularly to the surface area (Pressure=Force/Area). Air pressure is the collective result of the molecules that make up the air bouncing around and applying forces against any surface with which they have contact. The effects that we notice and attribute to air pressure are the result of balances and imbalances between air pressure in different areas or inside and outside of objects. For instance, an inflated ball remains inflated because the air pressure inside the ball is equal to or balanced with the air pressure outside the ball.
Why are concepts related to air pressure so difficult for students to understand? Many of the difficulties students have stem from assumptions students make about the nature of causes and effects that are very different from those that scientists make. These assumptions make it difficult for students to develop a deep understanding of air pressure and air pressure-related phenomena.1
This module introduces three causal forms that help students understand air pressure- related phenomena. These include recognizing and understanding: 1) non-obvious causes; 2) passive causes; and 3) relational causality. Understanding these causal forms influences students' ability to grasp a variety of pressure-related concepts such as weather patterns, Bernoulli's principle, and "vacuum" pumps, to name a few.
Non-obvious Causes
Scientifically accepted explanations often involve causes that are hard to notice. You can't observe the causes directly; you have to figure out that they exist. Many of the science concepts that give students difficulty involve non-obvious variables. For instance, students have difficulty reasoning about density (an intensive quantity),2 microbes and microbial recycling in ecosystems,3 and the process-like behavior of electrons and protons in electrical circuits, to name a few.4 Students need to know that causes can be hard to notice, or even impossible to see, so that they don't limit their search for explanations to obvious variables.
Air pressure is a non-obvious variable. It was not formally recognized until 1643 when the mathematician Evangelista Torricelli discovered that air pressure accounted for the height to which water could be pumped out of mineshafts.5 So it is no surprise that students and laypersons often fail to note air pressure's existence or contribution to an effect. Research shows that students ranging from 6 years old through university level fail to recognize air pressure when engaged in science activities focused on it.6 Students have difficulty shifting their focus from the apparent features of the task to less obvious variables, such as the behavior of air or water. Students' perception of how gases behave differs from that of scientists;7 and they have difficulty understanding gases and their role in air pressure.
Air is around us all of the time, so we are accustomed to the presence of air pressure. Beyond this, our bodies continually adapt to the sea of air in which we live. We are usually unaware of our bodies' adaptations; they only become obvious when air pressure changes rapidly, such as when our ears pop in an ascending airplane. When students are not aware of air pressure as a possible cause, they typically turn to concrete, obvious variables to try to explain pressure-related phenomena. For example, when asked why a balloon partially deflates when driving from higher to lower altitude, most students speculate about the possibility of obvious variables, such as a hole in the balloon, rather than changes in non-obvious variables, such as air pressure.
One way to help students recognize the existence of air pressure is to find ways to make its effects obvious. For instance, research has found that high school students do realize that the pressure of enclosed air in a syringe increases with compression.8 This is not startling since students feel the effect of the increased pressure on their hands. The effect is obvious. When the syringe was not compressed and they could not feel an effect, 70% of students thought the enclosed air did not have air pressure. Other research showed that 11- to 13-year-olds could not imagine air pressure without some type of movement associated with it.9 They considered equilibrium situations to be due to a lack of air pressure rather than due to equilibrium between pressures.
Passive Causal Agents
Closely related to the issue of the non-obviousness of air pressure is that of passive causal agency. When we envision causality, most of us picture some type of action leading to some type of effect. In this image, the cause is seen as active—it does something that leads to a result. However, some forms of causality do not neatly fit this image. Instead, they bring about outcomes (sometimes obvious and sometimes not) in ways that one might call passive. What do we mean by this? Here are some examples:
- An arch bridge is a feat of balanced forces. The stones that make up the arch distribute the weight around the half circle and the abutments maintain the shape of the half circle. The structure and resulting balance of forces causes the bridge to stay up. However, no active movement is associated with the outcome. If one of the abutments were to be removed, the role of the abutment in maintaining the arch and distributing the forces to keep the bridge up would become dramatically clear. However, we don't typically think of the abutments as "causing " something.
- When riding in a car at fifty miles per hour, everything in the vehicle is moving at 50 miles per hour, including us. The car has brakes to stop it. What stops us? Well, as long as the stopping of the car is gradual, we might not notice the role that our seatbelt plays in helping to stop us. It is a passive restraint system. When a car stops very hard (amplifying the effect), we are more likely to recognize our seatbelt as a cause that keeps us from lurching forward each time the car stops. A more active causal agent would have a greater chance of being recognized. For instance, if a hand came out of the dashboard and pushed passengers into their seats each time the car came to a stop, we would quickly recognize it as a causal factor in the system.
Passive causal agents are not easy to notice. They typically don't draw attention to themselves by dramatic outcomes. For instance, in the bridge example, the balanced forces just continue keeping the bridge up. The effect isn't noticeable; you could say that we take it for granted, and in this way, passive causes can be non-obvious. In the case of the bridge, the system is balanced. Passive causes are difficult to detect, unless the system is made active by disrupting its balance. We typically attach causality to events, so if the bridge fell down, we would say that something caused it to collapse, but we typically fail to realize the causes in play when the bridge stays up. When an event does occur, students typically look for active (and therefore more obvious) causal agents.
How does passive causality complicate matters for students? First, students are unlikely to recognize air pressure-related phenomenon that are not "event-like." Second, when they do attempt to reason about air pressure, they are likely to characterize it as an active causal agent—substituting notions of force for pressure. Let's consider each issue in turn.
Air pressure is an ambient variable that is typically always present. When air pressure is balanced, it doesn't appear to "do" anything. However, when the balance shifts, it results in events or changes (ears popping, balloons expanding, liquid rising, and so on) that make it appear active. Analogous to the bridge example above, disrupting the balance of the system results in a dramatic event that fits with students' expectations about what constitutes a cause and effect relationship (that something acts on something else to make something obvious happen).
Students typically characterize air pressure as an active push in one direction, a unidirectional force that pushes down. This could result from substituting a force conception for pressure. Or it could be a natural extension of students' understanding of pressure as the force per unit of surface area, where the force is applied perpendicularly to the surface area (Pressure=Force/Area). However, air pressure acts omni-directionally. Because particles are moving in all directions, every direction that we choose for a unit of surface area will have particles that hit it perpendicularly. Visualizing this requires students to take the simple case of one surface and extend it to all the possible surfaces that molecules could bounce off of. This is cognitively challenging!
In order to grasp that air pressure acts omni-directionally, students also have to realize that molecules that make up the air can bounce in all directions, not just down. Students often draw their models of air pressure unidirectionally, with pressure arrows pointing downward. They tend to think of air pressure as "pushing down on them" similar to the way they think of gravity pulling them towards Earth's surface. This is evident in students' conceptions of air pressure, and particularly recurrent with water pressure. As with air pressure, the idea of water pressure existing equally in all directions is counter-intuitive to students. 10,11
Pressure acting in a greater downwards direction than in a sideways one is more appealing to students. Even physics textbooks use terms that reinforce students' original erroneous conceptions of pressure.12 To further complicate matters, when students include other variables, such as gravity and the density of molecules that make up the air, in the equation, it reinforces the notion that air pressure must push down because it is subject to gravity. Without knowing how to factor the relative potency of the variables involved, students don't know how to reason their way out of these understanding traps.
One further source of students' confusion may generate from reasoning about air pressure related phenomena at different levels. We do speak of individual molecules that make up the air as forceful—bumping into and bouncing off of things. The ambient, omni-directional aspects of air pressure are due to the collective effect of individual molecules colliding and bouncing around. It is well documented that students have difficulty reasoning about collective behaviors, and moving back and forth between levels of individual interaction and collective, emergent outcomes.13 The problem can be cast in terms of reasoning about decentralized (or emergent) rather than centralized causes. A centralized cause typically has one agent that is primarily responsible for an outcome, for instance, one motorized floodgate that opens or closes to control water flow. A decentralized cause typically emerges from the behavior of many individual agents that behave according to individual rules, for instance, individual ants in an anthill pre-programmed to act in certain ways that ultimately give rise to the overall anthill organization. In the case of pressure, while molecules that make up the air bounce around and can be individually thought of as forceful and centralized, the collective behavior is ambient and decentralized. It can be difficult for students to coordinate these different characterizations when relating what happens at a molecular level with what happens at a collective level.14
Students' difficulties in understanding the passive, omnidirectional nature of pressure are particularly evident when students are learning about hurricanes. Even if students learn that winds are the result of air moving from areas of higher pressure toward areas of lower pressure, the wind moves in a specific direction and therefore, exerts force in that direction. Hurricanes have powerful winds and therefore indelibly imprint a forceful, unidirectional notion in students' minds. Students confuse the forcefulness of the direction of the winds with the omnidirectional, pressure-related cause of the wind. Hand in hand with this idea is the notion that faster moving wind is more powerful and therefore must exert more pressure (which directly contradicts Bernoulli's principle).15
Relational Causality
Many pressure-related concepts are explained by a relational pattern of causality. This means that an outcome is due to the relationship between two variables, either an equilibrium relationship or a differential between two variables (in this case, higher and lower pressure). Neither variable alone is the cause; instead, the interaction of the two must be considered. Contrast this with a simple linear model where one event directly causes a given outcome. A linear causal model is unidirectional and involves a one to one correspondence between causes and effects.16
Students typically use linear causal models to explain pressure-related phenomena. Research found that half of the 12-, 14- and 16-year-olds surveyed explained drinking from a straw in terms of pressure or a vacuum actively sucking or pulling, rather than a pressure differential between a lower pressure inside the straw and a higher pressure outside the straw as the cause of the event.17 (See graphics below.) The linear causal model has salience for students, perhaps because they view themselves as taking an active role in drawing the liquid through the straw.
An awareness of non-obvious causes and passive causal agency has the potential to move students towards a relational understanding of pressure-related phenomena. However, because simple linear causality is much easier to grasp and because most students are not familiar with relational causality, recognizing non-obvious and passive causes is not enough. Students benefit from explicit opportunities to learn about relational causality and discuss its role in pressure-related phenomena.18
| Linear Model: Sucking creates a vacuum in the straw that causes the liquid to go up it. —8th grader |
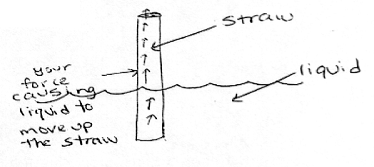 |
| Relational Model: When you drink from a straw the difference in the pressure inside and outside of the straw causes the drink to go into your mouth. When you suck on the straw, you draw air molecules from within the straw; the less dense air provides less pressure, especially compared to the air pressure outside of the straw. So the higher air pressure outside pushes the drink down deeper into the cup in the direction of the straw, and since that's the only opening the drink goes up the straw and into the mouth. —8th grader |
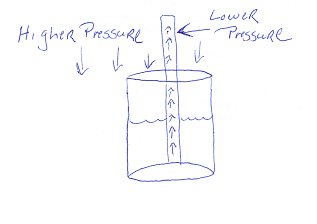 |
The examples above are about drinking from a straw. Students tend to reason linearly—you suck and it pulls the liquid into your mouth. Scientists reason relationally—removing some air from the straw lowers the air pressure in relation to the (now) higher atmospheric pressure, so that the liquid is pushed into the straw.
Breathing can also be described by relational causality. As you press upward on your diaphragm, the pressure inside your lungs is higher than the outside pressure and air flows out of your lungs. As you pull down on your diaphragm, the pressure inside your lungs is lower than the outside pressure and air flows into your lungs. Similarly, with a balloon, the pressure in the balloon is pushing out AND the pressure outside the balloon is pushing inward to give a balloon its shape. The balloon remains inflated because the air pressure inside it is equal to, or balanced with, the air pressure outside of it. Let's define the outside air pressure to be Po, and the inside air pressure to be Pi. When the outside air pressure (Po) = the inside air pressure (Pi), the pressures are balanced.
Often, however, instances occur which disrupt this balance with noticeable results. If you add more air to the balloon, it will inflate. Why? You need to look at it from two standpoints. First, consider what is happening inside the balloon. There are molecules that make up the air moving around quickly and bumping into the molecules around them. When you add more air to the balloon, you're adding more molecules, which do more bumping—therefore the force increases. Next consider air pressure outside of the balloon. It remains the same. This creates a pressure differential between the inside and outside of the balloon. Pressures always balance out (unless there is something preventing them from doing so, such as a lid on a jar). Thus the inside air pressure must change to be in equilibrium with the outside air pressure. How does it do this? The additional force within the balloon spreads out over a larger area. Remember that P=F/A.
In order for it to be in balance—and thus reach equilibrium—and for Po to equal Pi, the area of the balloon has to increase. The area increases (air pressure decreases) until equilibrium is reached between the inside and outside air pressures, with the result of a bigger balloon. If the area did not increase, the air pressure inside the balloon would increase because a larger force over the same area would result in a larger air pressure.
Other Challenges in Learning to Understand Air Pressure
This module introduces barriers to understanding air pressure that relate to how students think about the nature of causality. However, in our work with students, we have noticed other points of confusion in learning to reason about air pressure. For instance, students often take higher altitude to mean higher pressure, rather than lower pressure. While the confusion seems to be based more in linguistics than scientific understanding, it results in errors. Some of the confusions have to do with very basic concepts about the nature of air, for instance that it is matter and that it takes up space. Further, some of the activities that are typically part of pressure units can play into students' confusions. For instance, students think of air pressure as only pushing downwards. Activities such as the "bed of nails" (where someone, usually the teacher, lies down on a bed of nails) can be very effective for helping students understand the difference between force and pressure. However, the activity shows pressure, not air pressure and in helping students map its relevance to air pressure, there should be an explicit discussion of how pressure in this instance if different from air pressure. Issues such as these arise in any unit and with any activities. What's important is that teachers are alert to the sense that students are making and help students navigate around potential misunderstandings through discussion and targeted learning opportunities.
Summary
In summary, it is likely that your students' ideas will fall along a continuum of those presented here for each aspect of causality. A goal of this curriculum is to help students learn to reason about these three aspects of causality—non-obvious causes, passive causal agency, and relational causality—so that they can grasp the scientifically accepted explanations for pressure related phenomena.* Often, understanding one of these aspects supports understanding of the others; for instance, recognizing the non-obviousness of air pressure can bring students around to the relational model. If this happens with your students, they might see overlap within these lessons. Through the activities in this module, all students should make some progress towards models of air pressure that have greater explanatory power.
*From a philosophical stance, it should be recognized that some scientists refrain from attaching causality to concepts such as pressure that define parameters specifying the state of a system. They are more likely to talk about "how" a system behaves than "why" certain events take place and to focus on defining the laws to make it possible to predict what will happen. According to Physics Education Professor Josef Snir, rather than "pressure as a cause to different phenomena, pressure is a parameter that the scientific community defined in order to formalize rules and regularities in that set of phenomena." According to physicist Lester Chen (personal communication, April 30, 2003),
"The [little-known] nature of physics is that everything must be taken on a matter of faith. We [physicists] often talk about 'laws of nature,' but really, they are just descriptions or rules that we have come up with to describe the phenomena we observe. However, just because the rule described the last X experiences, that does not guarantee there is not another one coming up that proves the rule wrong (e.g., the often quoted theory of the ether). So, in the end, we are left with wondering "Why are the rules the way they are?"... There can be no satisfactory answer to this question. So, I prefer to stop the 'Whys' at the level of the rules."
Contrary to this stance, we do use causal language throughout this module and others. Our research shows that students bring and apply assumptions to their learning that are causal in nature and that these assumptions generate problems in developing deep understanding of the science concepts. Given that students already view the problems through a causal lens, we seek to substitute their problematic assumptions with ones that yield a better understanding of the science. Our research shows that doing so benefits students' understanding (of the concepts, if not the epistemology). Ultimately, it is our hope that those students who go on to study physics at the university level will have the opportunity to learn finer epistemic points about the nature of knowing in physics.


